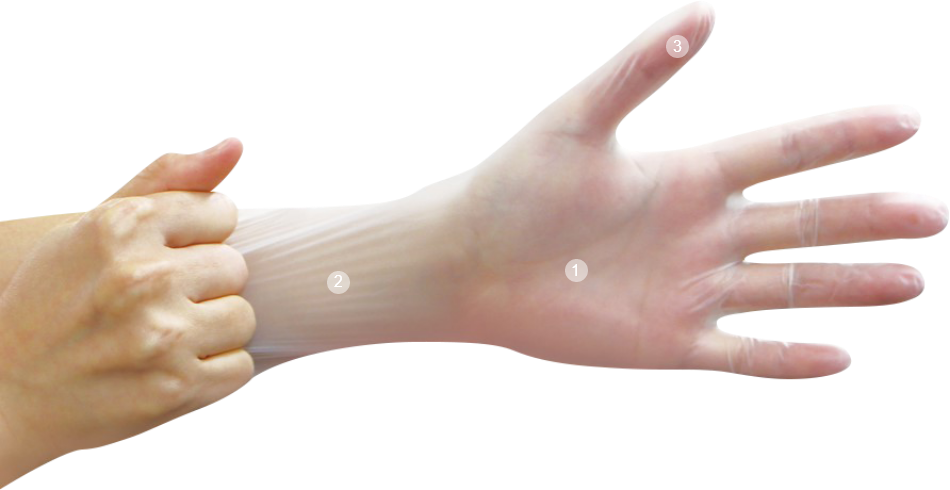
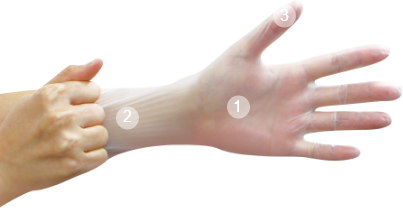
產品材質product material

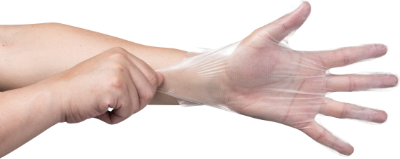
合成塑膠(PVC)手套
Vinyl Gloves
Vinyl Gloves
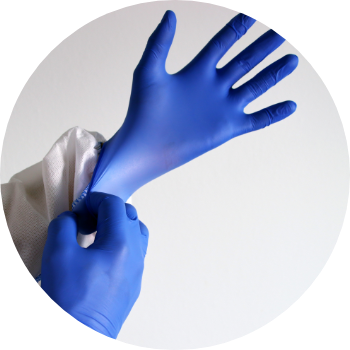
丁晴橡膠(NBR)手套
Nitrile Gloves
Nitrile Gloves

TPE 手套
TPE Gloves
TPE Gloves

5
全球工廠

17.4B
年產量

3,000+
全球員工

100+
全球市場
全球市場地區分佈Global footprint